Researchers in China have developed a method of salt water desalination that generates very little waste and is less susceptible to scaling. Their results have been published in the Journal of Membrane Science.
Only 0.01 percent of the Earth's water is fresh water. In contrast, 97 percent of the water on Earth is salt water, making seawater desalination an attractive option for meeting the global demand for fresh water.
Conventional seawater desalination can generate high-quality fresh water; however, it produces highly concentrated salt water which is usually discharged into the oceans, resulting in serious environmental and ecological problems. Furthermore, the build up of insoluble precipitates fouls the nanofiltration membranes used, making frequent replacement necessary.
By combining nanofiltration-based fractionation with electrodialysis-driven double displacement (metathesis), a research team from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of Chinese Academy of Sciences have developed a way to fully utilize the highly concentrated waste water.
Called fracsis (fractionation + electrodialysis), the process uses a commercially-available nanofiltration membrane to first separate ions with different valency. Valency is a measure of the ability to form covalent bonds. Divalent ions such as calcium (Ca2+) and magnesium (Mg2+) tend to form salts that dissolve poorly and clog up the membrane. To avoid this, the researchers first used a nanofiltration membrane to separate the monovalent and divalent ions in the high salt waste water.
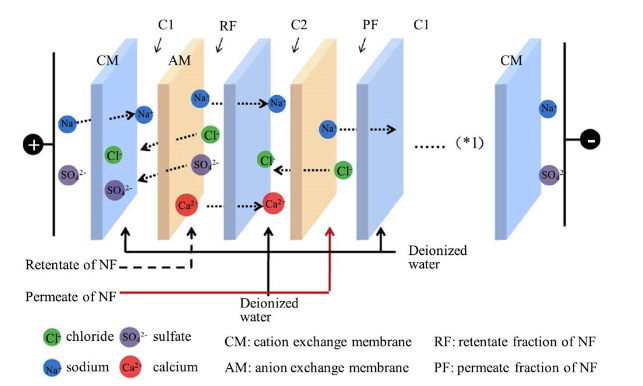
Figure 1. Model of electrodialysis for zero-discharge and valorization of seawater (Image by QIBEBT)
The separated solutions are then channeled to different electrodes for electrodialysis, where Mg2+ migrates to the direction of cathode and SO42- to anode. Both ions then move to adjacent compartments where they form highly stable salts through a process known as double displacement. This prevents the formation of precipitates of MgSO4, while producing high-purity drinking water at the same time.(Asian Scientist)

